Imagine a world in which Alzheimer's and Parkinson's disease, arthritis, blindness, and blood disorders are a thing of the past.
Unique cells known as stem cells could hold the key. Like magic seeds, they respond to built-in genetic instructions to develop into bone cells, muscle cells, brain cells or any other type of cell as the growing body takes form. Scientists believe these cells can also be used to treat many different diseases.
But research in this field has raised ethical questions, especially at some religious institutions. Researchers at a renowned Catholic university are working to find alternative ways to exploit the healing power of these extraordinary cells.
In the 1960s, two Canadian scientists discovered that stem cells from developing human embryos can regenerate tissue.
The finding raised the possibility of new treatments for diseases, like macular degeneration and Alzheimer's, in which body tissues are destroyed and the only cure is to replace those tissues.
Ethical conflict
However, these embryonic stem cells can only be harvested from living human embryos that have been either naturally or purposely aborted.
That creates an ethical conflict for people of many religious faiths, including Catholics, who consider embryos to be living beings, and their destruction, murder. It meant that researchers at universities with religious affiliations, such as Notre Dame in Indiana, could not explore this ground-breaking field.
So they looked for other ways to obtain and use stem cells that would not be morally problematic. They turned to adult stem cells, which come from mature cells.
Finding an alternative
Many researchers argued that adult stem cells were not as adaptable as those from embryos, but University of Notre Dame researcher David Hyde says they seem to be adapting quite well.
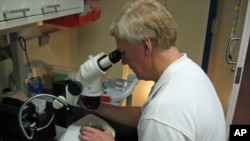
In a secure research facility, Hyde shows off a collection of more than 100,000 zebrafish. The two-and-a-half centimeter long blue striped creatures swim in their tanks, oblivious to the important role they're playing in retina research.
"All the fish are anesthetized before they're treated and most of the treatments involved use extremely bright light, which kills off their rod and cone photoreceptor cells in the retina," says Hyde.
Researchers know that the fish are blind because they do not swim away from simulated predators like their sighted counterparts. However, adult stem cells in the eyes of the zebrafish regenerate the damaged cells, restoring vision.
Hyde and his colleagues examine the stem cells under microscopes to try to determine how the regeneration process works.
Hyde says zebrafish have a similar eye structure to humans, but the stem cells in our eyes do not automatically regenerate.
Promising signs
"So we're ready to start to think about are these processes that we've identified in zebra fish, are they working properly in humans? Or is there something that is blocking their ability to function in humans, which would then correlate to the inability in the human retina to regenerate."
Ideally, Hyde and his colleagues hope to identify the mechanism that zebrafish use to regenerate their vision and apply the knowledge to humans.
Other stem cell researchers at Notre Dame are working with fruit flies to gain a better understanding of the biochemical processes in blood production, which is critical to curing blood diseases like leukemia or hemophilia. And mice are being used to research adult stem cells in bone, cartilage, and fat to gain answers in treating arthritis and orthopedic diseases.
According to Hyde, adult stem cells have proved even more useful than their embryonic counterparts in many ways.
"What we haven't been able to do is to take an embryonic stem cell, place it into a neural tissue such as the retina and have it become specific types of cells that regenerate only the damaged or missing cells," says Hyde. "That process of becoming a specific type of cell is going to be extremely difficult whereas these adult stem cells that already reside in that tissue seem to have already resolved that problem for us."
Cutting edge of adult stem cell research
While Notre Dame officials acknowledge that their university cannot compete with institutions like Harvard and Stanford in the field of embryonic stem cell research, it is on the cutting edge of adult stem cell research.
It's one of a growing number of American universities and institutions such as Wake-Forest in North Carolina, the University of Maryland, and the Mayo Clinic in Minnesota. Internationally, groups in Great Britain, Mexico, Singapore, Sweden and South Korea are also involved in adult stem cell research.
Notre Dame recently started an interdisciplinary adult stem cell initiative so faculty from its biology, law and engineering departments can work together in the area. Historian and philosopher Philip Sloan says the university is also looking into offering graduate training on adult stem cell issues to humanities, law, and science students.
"It's important to be aware of that not simply in spite of our religious affiliation, but because of a conception we have within the Catholic tradition of a need to interface science, theology, faith, and reason and these questions, I think those are going to be important signature that we can bring to this these discussions."
Next year, Notre Dame plans to host a workshop, bringing scientists and ethicists from around the country together to discuss the future of adult stem cell research.